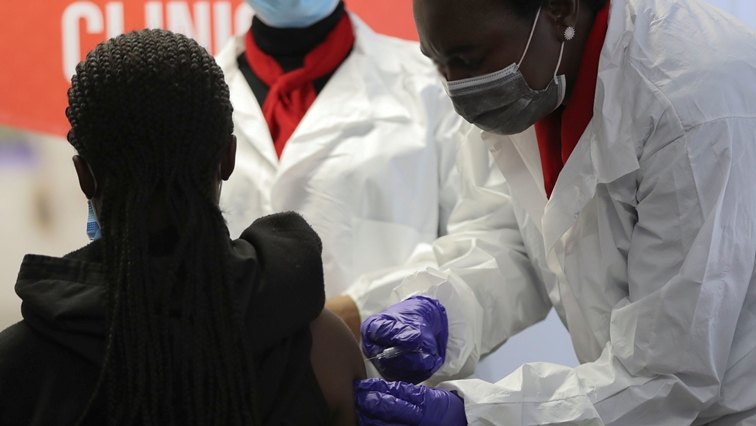
The South African Health Products Authority (SAHPRA), says it has approved the use of the Pfizer vaccine for children 12 years and over. The decision came after a review of updated safety and efficacy information submitted in March this year.
SAHPRA says on its website that this is in terms of Section 21 of the Medicines and Related Substance Act. Section 21 of the Medicines Act is a mechanism that enables emergency use access and also enables SAHPRA to authorise the use of medicine subject to certain conditions.
The two dose Pfizer vaccine has been shown to be highly effective in preventing severe illness and also preventing death from COVID 19.
After a bumpy start, South Africa’s vaccination campaign has ramped up in recent months with a solid supply of shots secured and just over 12% of its more than 60 million people vaccinated. That puts the country well ahead of others on the continent.
Latest SA stats:

Loading…
However, health insurers say vaccine hesitancy is now the main issue impacting the pace of the campaign and the government has launched efforts to persuade people to get the shot.
South Africa has a large youth population, with some 28% under the age of 15.
Countries around the world are now considering or administering vaccines to children, while other vaccine developers including Moderna are seeking the green light for the use of their shots on teens.
Pfizer, whose shot is already being administered to teens, is planning tests on even younger children.
SAHPRA authorises Sinovac’s CoronaVac shot for use: Dr Aslam Dasoo
–Additional reporting by Reuters.