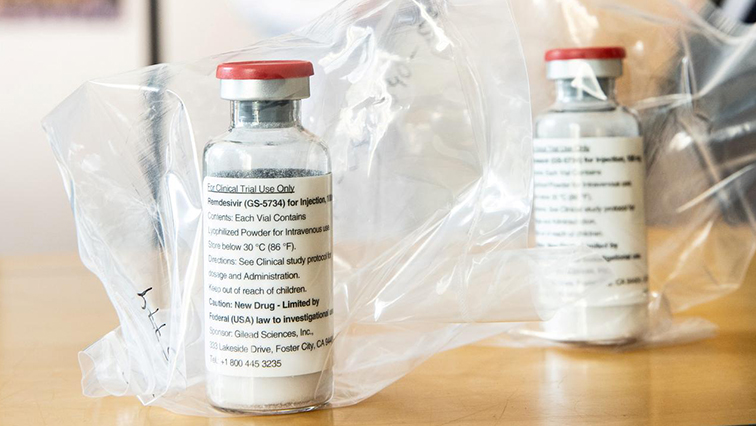
Pharmaceutical giant CIPLA has manufactured thousands of dosages of the anti-viral drug Remdesivir, which has been shown to shorten recovery time for COVID-19 patients.
CIPLA was granted a licence to manufacture after the drug was first tested by the United States’ Food and Drug Administration (FDA) in May.
The pharmaceutical giant now manufactures and distributes Remdesivir to 127 countries.
Cipla South Africa CEO Paul Miller says, “We’ve released close to 24 000 units to the market of Remdesivir. This is a product that can actually make a difference for those that are COVID-19 positive. And we hope that this can bring some help to the physicians who treat patients who are infected…”
“We will also continue to monitor the market and we should have another batch of 30 000 units readily available in the month of August.”
Medical researcher, Professor Helen Rees, gives an overview of what is known about Remdesivir
Food and Drug Administration’s overview of Remdesivir
According to the Food and Drug Administration (FDA), Remdesivir is an investigational drug, not approved by the FDA for any use. It is not yet known if it is safe and effective for the treatment of COVID-19.
The FDA has however authorised the use of the medicine in the United States under an Emergency Use Authorization (EUA) only for the treatment of patients with suspected or laboratory-confirmed SARS-CoV-2 infection and severe COVID-19.
An FDA statement says, “Remdesivir has demonstrated in vitro and in vivo activity in animal models against the viral pathogens that cause MERS and SARS, which are coronaviruses structurally similar to SARS-CoV-2, the coronavirus that causes COVID-19.”
Frequently Asked Questions about Remdesivir for COVID-19 patients: