Sixteen people freed after kidnapping at Nigerian university

Sixteen students and workers have been freed after they were kidnapped by gunmen who attacked their
Hamilton says next six months are crucial for Mercedes

An exhausted Lewis Hamilton wrestled his Mercedes to fifth place in the Japanese Grand Prix on
Ireland come out on top of bruising clash with South Africa

Top-ranked Ireland claimed a 13-8 statement victory over defending champions South Africa in a
4 SANDF members killed in Upington head-on collision, 2 critically injured

The South African National Defence Force (SANDF) says four SANDF members have been killed when two
Leaders in Zimbabwe need to find a lasting solution: Mbeki

Former President Thabo Mbeki says it is up to leaders in Zimbabwe to find a lasting solution to the
Portugal stack bench with forwards for Georgia match

Portugal coach Patrice Lagisquet has stacked his bench with six forwards to counteract Georgia’s
Legal professionals raise concerns over proposed amendments to RAF

The legal professionals have expressed concerns that the proposed amendments to the draft Road
Mbeki expresses confidence in resolving current electricity challenges in SA

Former South African President, Thabo Mbeki says the current electricity challenges in the country
Cashless taxi service launched in Cape Town

A taxi service that doesn’t require passengers to carry hard cash has officially been launched in
Over 200 displaced Harrismith residents plead for assistance

Over 200 displaced Harrismith residents in the Free State are pleading for assistance.
President Ramaphosa wraps up US visit

President Cyril Ramaphosa has concluded a working visit to the United States. He led the country’s
Libya floods sweep away migrants, and their hopes

The small first-floor apartment in Derna became home away from home to Syrian migrant Ammar Kanaan,
Three naval mariners perish near Kommetjie in Cape Town – NSRI

The National Sea Rescue Institute (NSRI) says three naval mariners have died following a rescue
Poor nations have ‘every right to be angry’ about climate crisis: UN chief

Developing countries demanded new financial help from wealthy nations in their uphill fight against
Rwanda’s President Kagame to seek re-election in 2024

Rwanda’s President Paul Kagame said he will stand for re-election next year, hoping to extend nearly
Over 40 schools become beneficiaries of Vitality Booster Porridge Programme in N West

Over 40 schools in the North West Province are beneficiaries of the Vitality Booster Porridge
World War One cemeteries, Rwanda genocide sites, Argentine torture centre declared UNESCO World Heritage

World War One cemeteries in Belgium and France, the hills of Rwanda’s 1994 genocide and a former
Now is the time to accelerate implementation in order to tackle climate change: Ramaphosa

President Cyril Ramaphosa says no country should ever have to choose between developmental
Rhino poaching remains a challenge in SA and Africa

As the international community marks World Rhino Day on Friday, South Africa and other parts of the
City Power disconnects electricity to hijacked buildings in Jeppestown
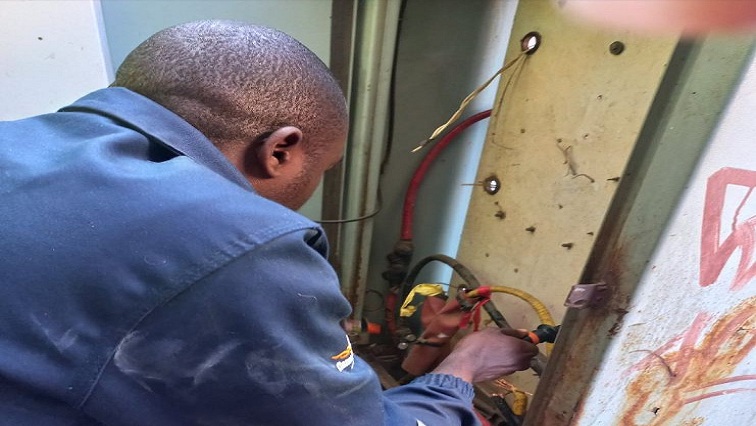
Johannesburg’s power entity, City Power has disconnected electricity to several hijacked buildings

