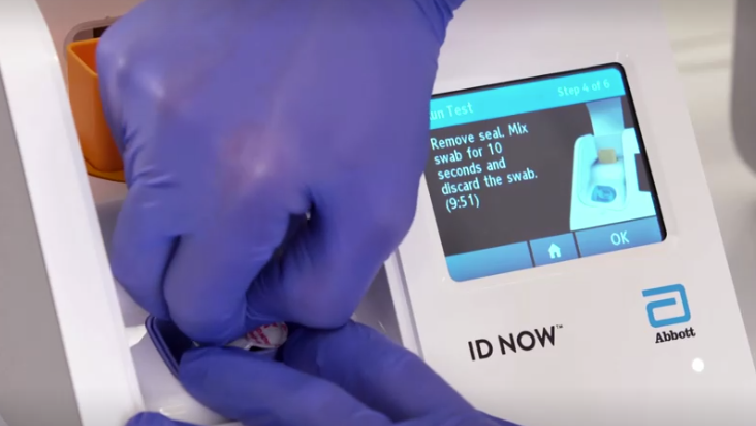
Abbott Laboratories said on Friday it won US marketing approval for a diagnostic test for the coronavirus that can deliver results to patients within minutes and be used in physicians offices and urgent care clinics, as well as hospitals.
The United States now has more cases of the coronavirus than any other country, and hospitals have struggled to meet the demand to test thousands of people for the often-deadly virus.
The US Food and Drug Administration granted approval under its Emergency Use Authorization.
Abbott said in a statement that it plans to begin distributing the test next week and will ramp up manufacturing to 50 000 tests per day.
According to laboratory officials, positive test results are available within 5 minutes and negative test results within 13 minutes.
The portable test will run on Abbott’s ID NOW platform.
It is the second test to be approved by the FDA that can be used directly in physicians’ offices and other community healthcare settings and promptly provide results to patients. Last week, the FDA approved a test made by Cepheid that can be used at the point of care.
Abbott received approval last week for a high volume, automated diagnostic test that can be used in laboratories and said at the time it would immediately distribute 150, 00 of the tests around the country.
Between the two platforms, Abbott said it plans to produce 5 million tests per month.
The FDA has been rushing to approve tests for the coronavirus on an emergency basis and has approved others made by companies including Roche Holding AG and Thermo Fisher Scientific Inc.
With the expansion of testing has come a surge in confirmed cases of COVID-19. Reuters reported on Friday that the United States now has more than 100 000 cases.