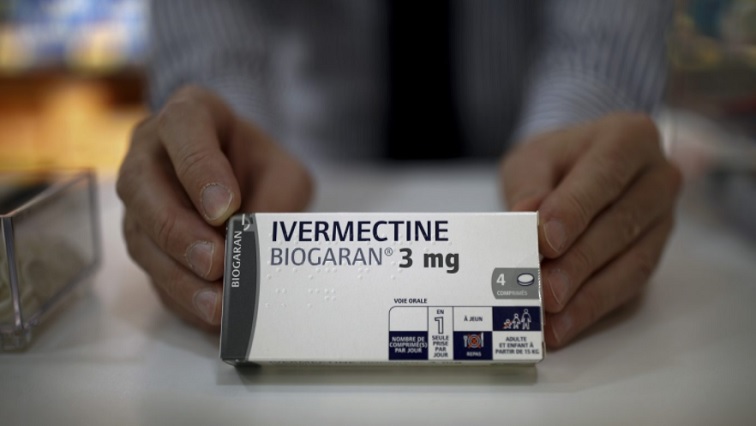
A study by the Soweto Clinical Trial Centre has shown that 80% of Ivermectin tablets found in South Africa contain at least one undeclared substance. According to the centre’s Managing Director, Dr. Qasim Ebrahim Bhorat, the ingredients could have dangerous consequences for patients.
The health products regulator, South African Health Products Regulatory Authority ( SAHPRA), recently approved the compassionate use of Ivermectin for the treatment of COVID-19 but under strict conditions.
Some health practitioners have been calling for the use of Ivermectin to treat coronavirus:
Health practitioners have been allowed to prescribe the drug under Section 21 Authorisation of the Medicines and Related Substances Act, even though the drug is not registered for human use in the country.
The Soweto Clinical Trial Centre has discovered that the drug could be detrimental to patients. Dr. Qasim Ebrahim Bhorat says that some of the ingredients found in the different formulations of Ivermectin are blood thinners, anti-depressants and sedatives.
He says many of these substances are not declared on the packaging and could be dangerous.
“Due to the absence of quality control and the illegal nature of these were brought in, there was no proper vetting and therefore the use of it would have uncertain safety effects on patients. I sourced seven different formulations of what was claimed to be Ivermectin. No one knew the quality of medication they were taking and there was no accountability in terms of who brought it in. When we tested these, they had seven undeclared formulations, not on the label of medications. Something we found was a blood thinner, another was an addictive, one was anti-convulsant.”
Safety of patients
Dr Bhorat says that healthcare professionals should not do as they please, especially with unregistered drugs. He says they have a responsibility to prescribe medicines that they can guarantee will not cause harm to patients. Dr Bhorat says undeclared substances in medication cast doubt on their safety.
“How do you prescribe and give someone something that you cannot vouch for? Each healthcare professional can’t just say but I bought it like this, it’s not registered in the country, you are taking a risk. You have to then say, I’m going to get it analysed, I know what it contains and I’m safely dispensing it. With unregistered medication, you don’t know what you are getting. That’s the bottom line. Even if you are a doctor – the use of Panado is legislated. You as a doctor are not allowed to do anything you want with any tablet – that’s ridiculous. So please just follow the law.”
Study on risks and benefits of Ivermectin
Doctor Maria Van Kerkhove, the World Health Organisation’s technical lead for COVID-19, says the WHO is reviewing data on global studies on the risks and benefits of Ivermectin.
“The clinical team is looking at data right now on different studies that have been evaluated on Ivermectin. They are synthesising the data from different studies. Some had small sample sizes and the idea is to pool all those together into a meta-analysis and apply it to assess the certainty, benefit, or risk-based on each of those studies. They have a steering committee and are following the results of clinical trials around the world and that is being used to trigger the development of the guidance by the WHO team.”
Illegal drugs
SAHPRA is stepping up its clampdown on illegal drugs and is working with SARS customs officials and the police to stop it from entering the country. Four people from India have already been arrested for smuggling the drug, Ivermectin, into South Africa.
The drugs, with an estimated street value of more than R6 million, were concealed in luggage. SAHPRA Regulatory Compliance Manager, Daphney Fafudi, says no-one should be in possession of the drug without their consent.
“We have been getting reports from the public, social media platforms, customs, police regarding contravention. We sent an inspector and found an undeclared shipment which was nicely packed – inside was Ivermectin. This was at OR Tambo. The three were arrested and are currently out on R50 000 bail. In Durban, also undeclared personal luggage, it was an Indian national as well. This one is out on R5 000 bail.”
The Soweto Clinical Trial Centre says that without SAHPRA approval, the quality of available Ivermectin tablets remains uncertain. They say that using medication that has bypassed South African quality control processes, raises significant safety concerns and has legal ramifications for the prescribing doctor.